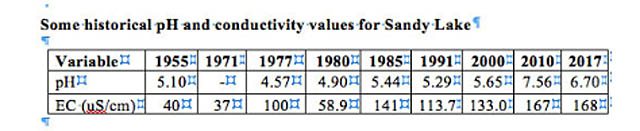Revised Dec 17, 2018
On December 13, I repeated a set of observations on pH (a measure of acidity) and EC (electrical conductivity, a measure of salt content) that I had made just over a month ago (Nov 8, 2018) on streams entering Sandy Lake via culverts at the southwest corner.
The lake was frozen solid. I am told it is the first time it has frozen over before January in about 20 years.
Electrical conductivity of Sandy Lake itself has increased over the years:
The increase can be attributed to increased urbanization within the watershed and associated use of horticultural fertilizers and road salt. The levels are not high enough to be directly toxic, but there is a worrisome trend for the deeper waters to have higher conductivity (i.e to be saltier) than the water at the surface of the lake; if that trend continues it could impede seasonal turnover of the deep water, which is essential to the health of the lake. Also, while electrical conductivity is due to salts in the water, it is likely that there is organic loading as well coming from the urbanized areas. (View Lakes for more on this topic.)
I routinely measured electrical conductivity of streams as I encountered them when I was conducting extensive field surveys in 2017. Those data and also a survey of values around Sandy Lake indicated that the major source of salt input to Sandy Lake was Johnson’s Brook, entering at the southwest corner. (View maps under Lakes > Ec & pH.) However, in 2017, I did not made any observations on electrical conductivity and pH on the streams that converge on Johnson’s Brook. On Nov 8, 2018 I conducted such observations and it is those observations that I repeated on Dec 13, 2018.
The results for Nov 8 and Dec 13 are shown below on Google Earth maps for the area.
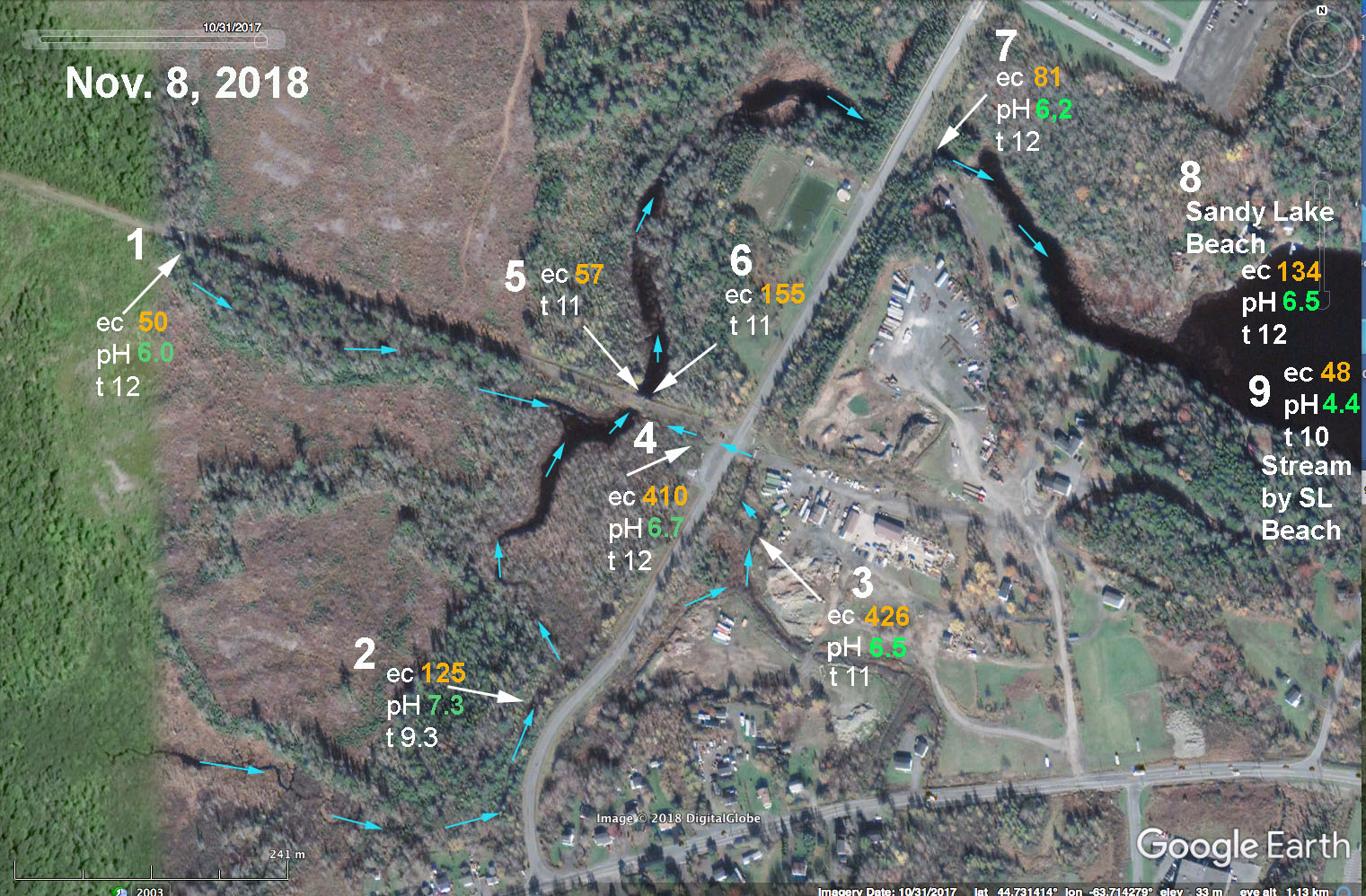
Above: EC and pH values for streams associated with Johnson’s (Bob’s) Brook on Nov 8, 2018. #1 Upper Johnson’s Brook. #2 Brook draining from Uplands Park wastewater treatment area. #3 and # 4: Brook draining construction/trucking yard and community just to the SE of the Dairy Road. These 3 system converge in the area of sites 5& 6 and then water flows downstream to enter Sandy Lake through two large culverts at site #7 (still known as Johnson’s Brook). Nos 8 and 9 were taken at Sandy Lake Beach Park for reference. There were strong flows in the brooks at sites 1 and 2. A steady but weaker flow was observed at site 3-4 (it crosses the road from 3 to 4 through a culvert and that water was very cloudy and had a lot of suspended material.
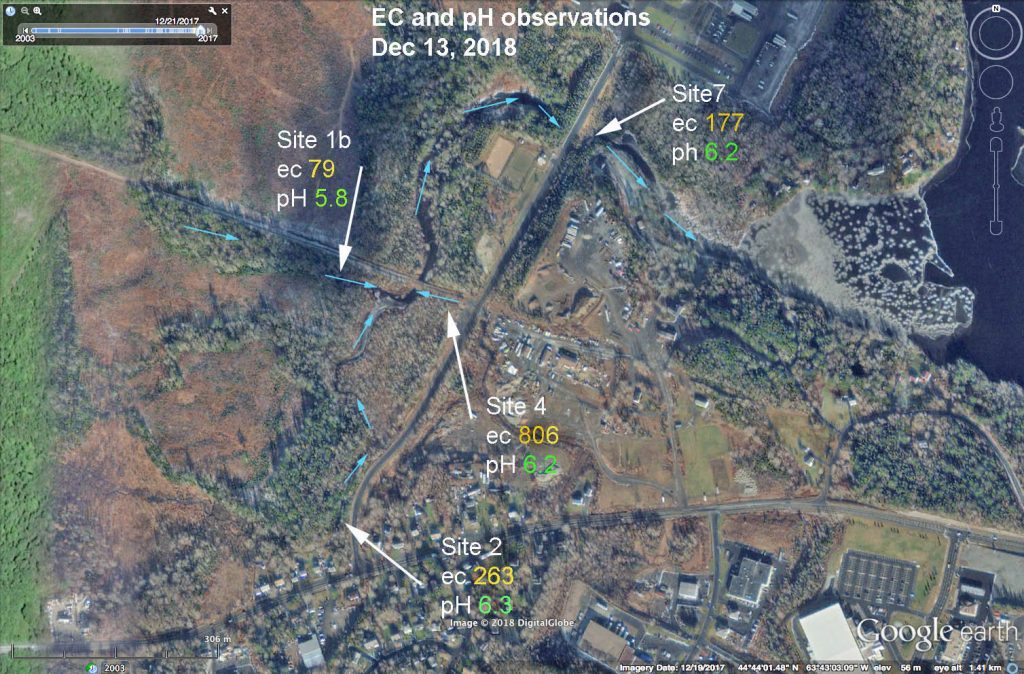
EC and pH values for streams associated with Johnson’s (Bob’s) Brook on Dec 13, 2018. EC (electrical conductivity) and pH values of samples are the values after samples had warmed to room temperature (18.5 deg C)
Between Nov 8 and Dec 13, electrical conductivity values increased in each of the three streams that converge before the water enters Sandy Lake, values for two of the three streams approximately doubling, and the other increasing by about 50%. EC values for the two dates are highlighted in red in the table below. The increase can be attributed to the initiation of road salting between those two dates as the cold weather set in. So the increase can be described as a “salting signal”.
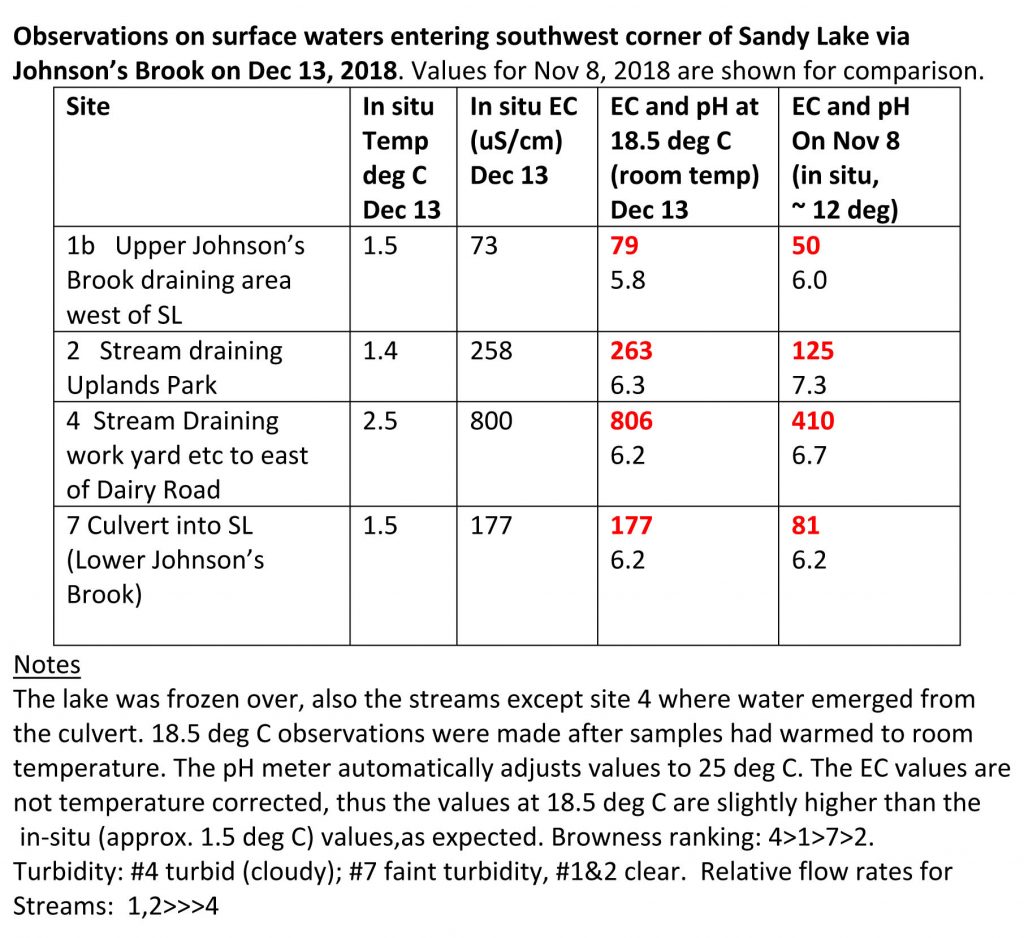
The flows on Dec 13 were definitely lower than on Nov 8, I would guess by a factor of 2 to 3, which is due to freezing of the ground and precipitation falling as snow rather than rain.
I expect that the peak salt input to the lake will occur when temperature starts to rise in the spring, the lake to melt and the surrounding lands to thaw, especially if there has been a lot of salting over the winter. It’s then that the incoming water could get very briny (e.g. EC 1000 uS/cm and above), and move in a layer to the bottom of the lake.
For more about the surface waters associated with Sandy Lake, view
– sandylakebedford.ca > Lakes
– sandylakebedford.ca > Lakes > Limnological Profiles
– sandylakebedford.ca > Lakes > EC & pH
To see an example of a local lake affected by multiple factors including heavy roadsalt loading, View
Oathill Lake Lake Restoration
Slide Presentation from the Oathill Lake Conservation Society, 2018.
Also: Oathill Lake Conservation Society on Twitter (lots of Photos)
It’s a remarkable story of how citizens got together to restore the lake to the extent possible given that the area is heavily settled. A lot of what they have begun to get back, with a lot of effort, are features Sandy Lake still retains. We need to ‘work on it’ to keep it that way, also to restore the lake to a more pristine condition.
Some literature on lakes and road salt
Novotny, E.V and Stefan, H.G. 2012. Road Salt Impact on Lake
Stratification and Water Quality. Journal of Hydraulic Engineering 138:
1069-1080.
Road Salt Effects on the Water Quality of Lakes in the Twin Cities Metropolitan Area
Novotny et al. 2007. ST. ANTHONY FALLS LABORATORY Engineering, Environmental and Geophysical Fluid Dynamics Project Report No. 505
Sibert, R.J. et al., 2015. Cultural meromixis: Effects of road salt on the
chemical stratification of an urban kettle lake. Chemical Geology 395:
126-137.
Koretsky, C.M et al. 2012. Redox stratification and salinization of three
Kettle Lakes in Southwest Michigan, USA. Water Air & Soil Pollution
223:1415–1427.
Water quality measurements on Williams Lake and Colpitt Lake (Halifax, N.S.) Dec 7-13, 2015 with reference to possible impacts of road salt
Report to Williams Lake Conservation Company (WLCC) by David Patriquin January 6, 2016
———-
Water Colour
Water samples Dec 13, 2018. The “brownness” decreases in the order A, B, C, D. Numbers refer to the sites (see map above)
Instruments used to measure pH and EC