These Items will be posted on other pages (eventually)
Differential effects of freshwater browning across fish species: consequences for individual- to community-level fish traits in north temperate lakes
Allison M. Roth et al., 2025 in Biological Reviews
From the Intro: Inland waters are home to a disproportionately large diversity of fishes, hosting 40% of the world’s fish species, despite representing less than 1% of all water on the planet (Dudgeon et al., 2006; Likens, 2009). Unfortunately, fresh waters are increasingly exposed to multiple stressors, including climate change, alterations in land use, and species invasions, all of which vary by region (Birk et al., 2020; Griffiths et al., 2022). The darkening of fresh waters – hereafter ‘browning’ – is one form of environmental change that has been altering many physical, chemical, and biological attributes of freshwater habitats over the past few decades, especially those in northeastern North America and northern Europe (Monteith et al., 2007; Garmo et al., 2014; Solomon et al., 2015; Meyer-Jacob et al., 2019; Anderson et al., 2021; Räike et al., 2024). Analyses of water-column time-series data from 49 eastern Canadian lakes have revealed regionally specific trends (Imtiazy et al., 2025). Specifically, areas with historically intense acid deposition appear to have increased in browning from the late 1980s until ~2010, followed by a stabilizing trend or slight decline (Imtiazy et al., 2025). By contrast, a more remote area has demonstrated a pronounced increase in browning since ~2015 (Imtiazy et al., 2025).
Browning is most often caused by increased concentrations of terrestrial dissolved organic carbon (DOC; Monteith et al., 2007; Kritzberg, 2017; Anderson et al., 2021), although augmented iron levels may also cause browner waters (Kritzberg & Ekström, 2012; Lebret et al., 2018; Anderson et al., 2021). Anthropogenic stressors such as climate change (Weyhenmeyer & Karlsson 2009; de Wit et al., 2016; Meyer-Jacob et al., 2019), land use change (Meyer-Jacob et al., 2015; de Wit et al., 2016; Finstad et al., 2016; Kritzberg, 2017), decreased atmospheric acid deposition (Monteith et al., 2007; Clark et al., 2010; de Wit et al., 2021), and heightened nitrogen deposition (Rowe et al., 2014; Sawicka et al., 2017) can increase terrestrial DOC export, resulting in browning. By altering both chemical and physical properties of freshwater ecosystems (e.g. oxygen availability, light attenuation, and nutrient availability), browning may trigger ecosystem responses across spatiotemporal, biological, and ecological scales (Fig. 1; Solomon et al., 2015; Albrecht et al., 2023). For instance, Sherbo et al. (2023) found that browner lakes within the Experimental Lakes Area in Ontario, Canada (N = 286) had shallower euphotic and thermocline depths. Furthermore, within a subset of these lakes, browner lakes had lower gross and net primary productivity rates in the euphotic zone (Sherbo et al., 2023). Browning may also influence pelagic–benthic energy pathways (e.g. Vasconcelos et al., 2018, 2019; Koizumi et al., 2023), and there has been considerable effort dedicated to studying the effects of browning at lower trophic levels, such as plankton (reviewed in Creed et al., 2018; Blanchet et al., 2022). For example, Tanentzap et al. (2017) compiled stable isotope data from 147 lakes to show that the median relative contribution of terrestrially derived organic matter to zooplankton biomass was 42% and increased with DOC concentration.
Despite fish being an important structuring force in fresh waters, most current evidence detailing the effects of browning on fish is either species specific, lake specific, or experimental.
From the Abstract: From our literature synthesis, we found that fish growth is often negatively associated with browner waters, despite browning generally showing no effect on fish foraging. We also demonstrated that browner waters had greater abundances of northern pike (Esox lucius) and walleye (Sander vitreus), but lower numbers of lake trout (Salvelinus namaycush), yellow perch (Perca flavescens), largemouth bass (Micropterus salmoides), smallmouth bass (M. dolomieu), and lake whitefish (Coregonus clupeaformis). Moreover, we showed that fish communities were significantly more likely to contain species with larger eyes in browner lakes. Lastly, we examined relationships between various metrics of browning (i.e. dissolved organic carbon, Secchi transparency, water colour) and present a framework for how the effects of freshwater browning on fish may scale from individuals to populations to communities.
Lake Ecology Primer
Concise introduction to physical, chemical and biological aspects of lakes, by Water on the Web, a National Science Foundation funded project.
PHOSPHORUS: CANADIAN GUIDANCE FRAMEWORK FOR THE MANAGEMENT OF FRESHWATER SYSTEMS
Canadian Water Quality Guidelines for the Protection of Aquatic Life, Canadian Council of Ministers of the Environment, 2004
Predicting trophic response to phosphorus addition in a Cape Breton Island lake
Kerekes, Joseph J.1983. Proceedings of the Nova Scotian Institute of Science, 33(1), 7-18.
Schwarts, P. Y., Underwood, J. K. (1986). Lake classification in Nova Scotia from phosphorous loading, transparency and hypolimnetic oxygen consumption. Proceedings of the Nova Scotian Institute of Science, 36(1), 13-2 Three indices of eutrophication are used to compare effects of urbanization on seven headwater lakes near Halifax, Nova Scotia. Annual (1983) inputs of phosphorus were calculated and compared with lake Secchi transparencies and rates of consumption of hypolimnetic oxygen (Thienemann index). Results from transparency and oxygen deficits were similar but implied greater eutrophication than did the phosphorus index. Brief discussion of some inherent problems of each index is included.
Underwood, J. K., and P. Y. Schwartz. “Estimates of the numbers and areas of acidic lakes in Nova Scotia.” Proceedings of the Nova Scotian Institute of Science. 39.1 (1990): 11-18. There are 6674 lakes larger than 1 hectare in Nova Scotia covering an area of 2255 kml. Geological and pH isopleth maps were consulted to estimate acidified and acid sensitive lakes. Assuming that granitic or metamorphic bedrock only very slowly produce add neutralizing ions, we estimate that 78% of the lakes (65%of la ke area) would, in the absence of moderating influences of surficial geology and marine aerosols,be susceptible to acidifjcation. When all sources of acid neutralizing capacity are indirectly considered via examination of pH isopleths drawn from lake chemistry, we estimate that 16% of the lakes \26%01 lake area) have zero alkalinity. and that 69% of the lakes (80% of lake area) have < 50 J,teq l- alkalinity.
Factors influencing flux, mineralization and availability of phosphorus in clear and organic lakes in the Atlantic Region.Beauchamp, Stephen 1990 MSc thesis. The availability of phosphorus is especially important because of its role as the primary limiting nutrient controlling the epilimnetic productivity of many lakes. Grahn et al. (1974) were the first to suggest that the continuous input of acidifying substances into aquatic ecosystems might lead to reductions in lake productivity, and coined the phrase “oligotrophication”. Their conclusions were based, in part, on the loss of more alkiphilic plant species (i.e. Lobelia sp. and Isoetes sp.) from the benthic plant community and the subsequent expansion of Sphagnum sp.
Strong, K.W. (1987). The effect of lime on the zooplankton population of Sandy Lake, Halifax County, Nova Scotia. Proceedings of the Nova Scotian Institute of Science, 37(2), 63-70.
Hydrological and morphometric data for Sandy Lake are included in Table I. Prior
to liming, water chemistry of Sandy Lake was typical of lakes on metamorphic rocks in Halifax County (Watt et al. 1979; White et al. 1984). The watershed lies entirely on metamorphic rock of the Goldenville Formation. The lake is fed by two small brooks, and there was no evidence of organic pollution. Surface pH before the addition of lime varied from 4.1-5.1, varying little with depth. Gorham (1957) reported a pH of 5.02-5.18 for Sandy lake in December, 1955, and Watt et al. (1979) remeasured the pH in January, 1977, and reported values of 4.5&-4.58.
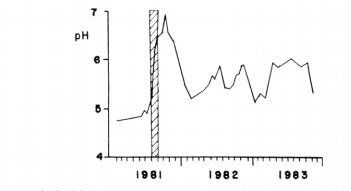
lake pH measurements (average of pH’s of all depths sampled—-depths usually included 0, 1,3,6, 12,16 and 18 m) are presented in Fig 1. The pH increased after the lime application from about 4.9 in August to 6.9 in October, 1981, followed by a decline to a post liming minimum of 5.2 in February of 1982. The pH varied thereafter from 5.4 to 5.8, declining to 5.1 by January, 1983. A similar pattern was repeated during the remainder of 1983. The pH did not return to the low values recorded during the pre-liming period. White et al. (1984) determined that pH increases in 1982 following the February1982 minimum were largely due to increased pH levels in oneof the two inlet streams (their Fig 5); I assume that the same factor contributed to increases during 1983.
The Chemical Composition of Lake Waters in Halifax County, Nova Scotia
E. Gorham 1957 in Limnology & Oceanography. Sandy Lake was sampled.
Establishing realistic management objectives for urban lakes using paleolimnological techniques: an example from Halifax Region (Nova Scotia, Canada)
Ginn ey al., 2015 Lake and Reservoir Management Volume 31 Of our 51 lakes, 22 (including some experiencing pH, TP, or showing floristic changes linked to climate changes) had increases in measured conductivity (1980–2002) and, correspondingly, increased relative abundances of halophilic diatom taxa. These lakes, often with catchments containing high surface areas of impervious surfaces, are examples of a trend of increasing salinity in northeastern North American lakes likely related to winter application of deicing (road) salt. The application of this paleolimnological approach enabled us to identify which lakes have undergone significant changes in diatom assemblages, as well as which environmental stressor(s) were most probable. This information can help lake managers develop more targeted and effective management strategies.
www.lakes.chebucto.org/
A website developed by Shalom M. Mandaville who has been very active in relation to aquatic issues locally over many years; he led formation of the Soil & Water Conservation Society of Metro Halifax (SWCSMH) “founded on the express encouragement of the Hon. John Leefe and senior staff of the Nova Scotia Environment Department in 1989.” A lot of info on this site.
Dynamic Environment & Ecosystem Health Research Resources & Links
St.Mary’s University
